CytomX Therapeutics Announces Third Quarter 2020 Financial Results and Provides Business Update
“As we approach the end of the year, the productivity of our R&D engine is evident with the growing breadth of our clinical pipeline, which is increasingly focused on previously undruggable targets. Between CytomX and our partners, there are now five Probody programs in the clinic, with four in Phase 2 studies, including CX-2029, for which we were excited to announce the treatment of the first patient in the expansion cohorts earlier today,” said
THIRD QUARTER BUSINESS HIGHLIGHTS AND RECENT DEVELOPMENTS
Clinical Pipeline Progress: Advancing into Multiple Phase 2 Expansion Cohorts
CX-2009: Phase 2 Expansion Studies in HER2 Negative Breast Cancer Subtypes in 2020
- CytomX expects to initiate a redesigned, multi-arm Phase 2 study evaluating CX-2009, a first in class anti-CD166 Probody drug conjugate, in patients HER2 negative breast cancer, in the fourth quarter of 2020. CX-2009 is armed with the maytansinoid payload DM4.
- This Phase 2 study is expected to be comprised of three arms. Arm A will enroll patients with hormone receptor (ER, PR) positive, HER2 non-amplified breast cancer for treatment with CX-2009 monotherapy (7mg/kg, q3w). Arm B will enroll patients with triple negative breast cancer (TNBC) for treatment with CX-2009 monotherapy (7mg/kg, q3w). Arm C will enroll patients with TNBC for treatment with CX-2009 monotherapy (7mg/kg q3w) in combination with CX-072 (pacmilimab, 1200mg q3w), the Company’s proprietary anti-PD-L1 Probody therapeutic candidate. Additional information is available at ClinicalTrials.gov using the identifier NCT04596150.
- Data from the Phase 1 study of CX-2009 in patients with HER2 negative breast cancer were presented at the American Society of Clinical Oncology’s (ASCO) ASCO20 Virtual Scientific Program in May and will be updated at
San Antonio Breast Cancer Conference later this year.
CX-2029: Initiation of Phase 2 Expansion Cohorts
- Today, CytomX, in partnership with AbbVie, announced the treatment of the first patient in the Phase 2 expansion cohorts evaluating CX-2029, a first in class anti-CD71 Probody drug conjugate, as monotherapy in patients with head and neck squamous cell cancer (HNSCC), squamous non-small cell lung cancer (SqNSCLC), esophageal carcinoma, and diffuse large B cell lymphoma (DLBCL). Additional information is available at ClinicalTrials.gov using the identifier NCT03543813. CytomX anticipates initial data from this study in late 2021.
- Preliminary clinical data from the first-in-human, Phase 1 dose escalation study of CX-2029 in patients with solid tumors was presented at the American Society of Clinical Oncology’s (ASCO) ASCO20 Virtual Scientific Program in May.
- Updated data from an
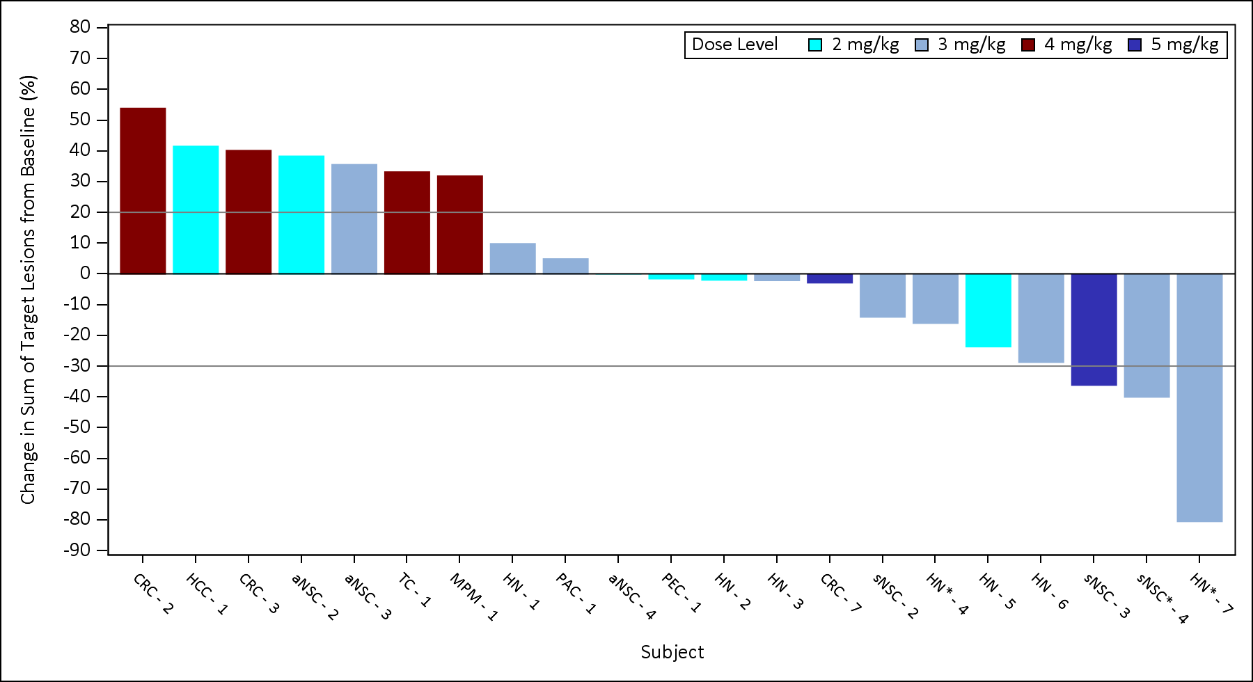
August 14, 2020 cutoff of this study is included below: (Figures 1 and 2). Of note:- 12 patients with sqNSCLC or HNSCC were enrolled in the Phase 1 dose escalation
- 4 patients with sqNSCLC were enrolled into the 1, 3 or 5mg/kg cohorts of the study.
- 3 out of 4 patients had a best response of stable disease or better:
- 2 patients had confirmed partial responses with a duration of 2.5 months and 5.6 months, dosed at 5 and 3 mg/kg respectively.
- 1 patient with stable disease enrolled in the 3mg/kg cohort remained on treatment with stable disease for 26 weeks.
- 1 patient experienced disease progression at the first on-treatment assessment and was enrolled at the 1mg/kg dose level.
- 3 out of 4 patients had a best response of stable disease or better:
- 8 patients with HNSCC were enrolled into the 2 or 3mg/kg cohorts of the study.
- 7 out of 8 patients showed a best response of stable disease or better:
- 1 patient with a confirmed PR remains ongoing at 38 weeks on treatment, with target lesion reduction of greater than 80%.
- 1 patient with stable disease remains on treatment at 33 weeks.
- The remaining patients have come off treatment for disease progression.
- 7 out of 8 patients showed a best response of stable disease or better:
- None of these 12 patients stopped treatment for a toxicity related issue.
- 4 patients with sqNSCLC were enrolled into the 1, 3 or 5mg/kg cohorts of the study.
- The most commonly occurring Grade 3 or higher adverse event was anemia, occurring in 49% of 45 treated patients across all dose levels. No new safety signals were observed at the updated data cutoff.
- 12 patients with sqNSCLC or HNSCC were enrolled in the Phase 1 dose escalation
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/d7d7c754-e600-4de7-8f77-d12d0605f2df
Figure 1: CX-2029 Spider Plot: Phase 1 Dose Escalation Study (2-5 mg/kg)
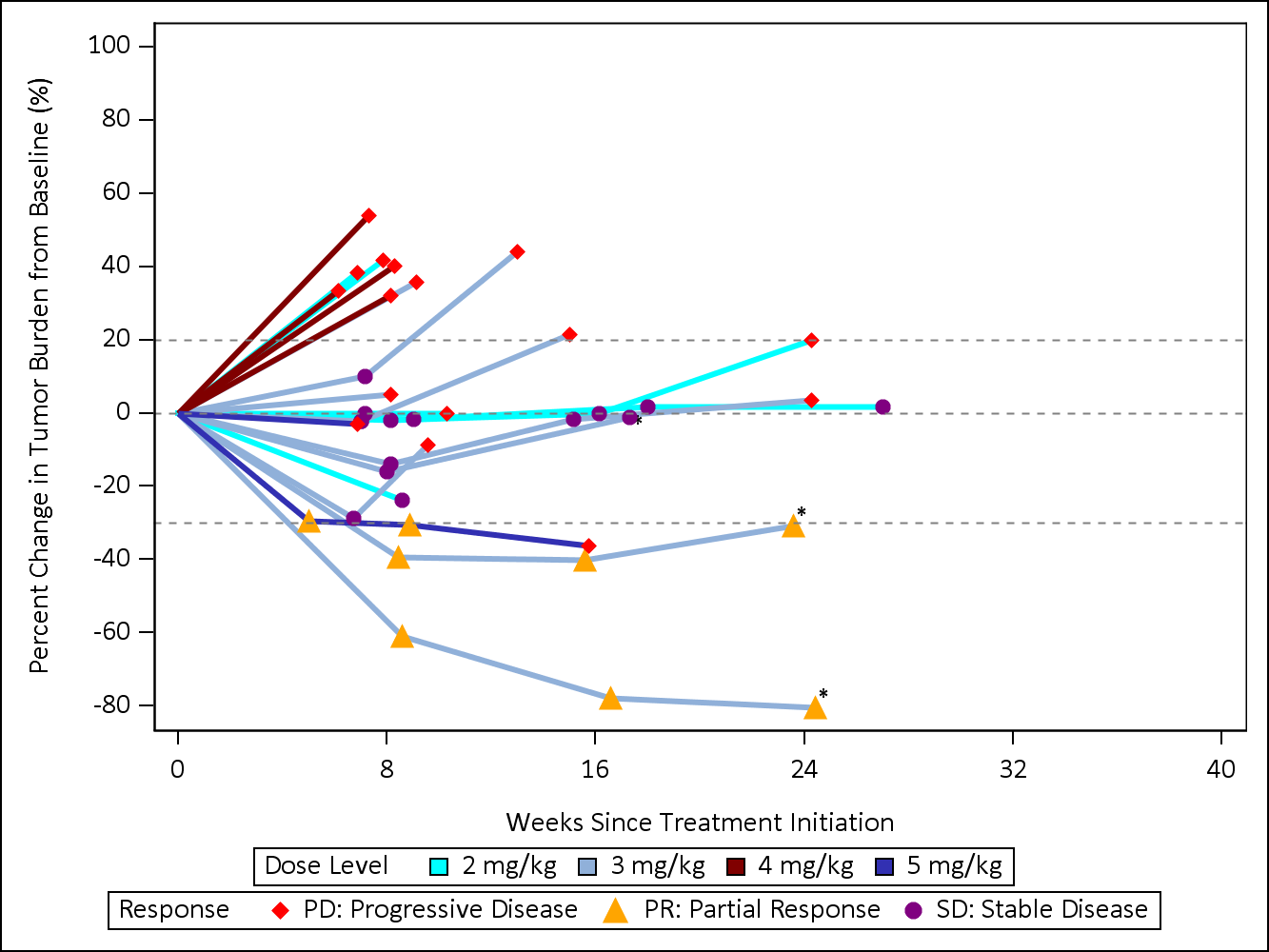
Figure 2: CX-2029 Waterfall Plot: Phase 1 Dose Escalation Study (2-5 mg/kg)
https://www.globenewswire.com/NewsRoom/AttachmentNg/7cd5e6cb-e0dd-4e3d-b69d-217f3f90babe
BMS-986249: Anti-CTLA-4 Probody Immunotherapeutic
- Bristol Myers Squibb continues to enroll patients as part of the Part 2a randomized cohort expansion of the ongoing Phase 1/2a trial of BMS-986249 administered in combination with nivolumab (Opdivo®) in patients with metastatic melanoma. Additional information is available at ClinicalTrials.gov using the Identifier NCT03369223.
- Bristol Myers Squibb presented safety data from the dose escalation stage of a Phase 1/2a trial at the American Society of Clinical Oncology’s (ASCO) ASCO20 Virtual Scientific Program in May.
BMS-986288: Anti-CTLA-4 Non-Fucosylated Probody Immunotherapeutic
- Bristol Myers Squibb continues to enroll patients as part of the Part 1 dose escalation study of the ongoing Phase 1/2a trial of BMS-986288 administered as monotherapy and in combination with nivolumab in patients with selected advanced solid tumors. Additional information is available at ClinicalTrials.gov using the Identifier NCT03994601.
Preclinical Pipeline
CX-2043 EpCAM Probody Drug Conjugate
- In October, CytomX presented updated preclinical data at the 32nd EORTC-NCI-AACR Symposium for CX-2043, a Probody Drug Conjugate targeting EpCAM (Epithelial Cell Adhesion Molecule), a widely expressed tumor antigen. CX-2043 is conjugated to the novel maytansinoid payload, DM-21. CX-2043 demonstrated potent anti-tumor activity across multiple cancer types and superior tolerability in animal models compared to the corresponding antibody drug conjugate. CytomX is advancing CX-2043 towards clinical studies with IND filing projected for late 2021.
CX-904 EGFR-CD3 Probody Bispecific
- CytomX continued to advance CX-904, the lead candidate from the Epidermal Growth Factor Receptor-CD3 T-Cell Bispecific program, towards IND-enabling studies. CX-904 is partnered with Amgen as part of a global co-development agreement.
Third Quarter 2020 Financial Results
Cash, cash equivalents and short-term investments totaled
Revenue was
Research and development expenses decreased by
General and administrative expenses were essentially flat during the three months ended
Teleconference Scheduled Today at 5:00 p.m. ET (
Conference Call & Webcast Information
CytomX management will host a conference call today at
About CytomX Therapeutics
CytomX is a clinical-stage, oncology-focused biopharmaceutical company with a vision of transforming lives with safer, more effective therapies. We are developing a novel class of investigational antibody therapeutics, based on our Probody® technology platform, for the treatment of cancer. CytomX has strategic drug discovery and development collaborations with AbbVie, Amgen, Astellas, and Bristol Myers Squibb.
Probody therapeutics are designed to remain inactive until they are activated by proteases in the tumor microenvironment. As a result, Probody therapeutics are intended to bind selectively to tumors and decrease binding to healthy tissue, to minimize toxicity and potentially create safer, more effective therapies. As leaders in the field, our innovative technology is designed to turn previously undruggable targets into druggable targets and to enable more effective combination therapies. CytomX and its partners, comprised of leading biotechnology and pharmaceutical companies, have developed a robust pipeline of potential first-in-class therapeutic candidates against novel, difficult to drug targets and potential best-in-class immunotherapeutic candidates against clinically validated targets. The CytomX clinical stage pipeline includes first-in-class product candidates against previously undruggable targets, including a CD166-targeting Probody drug conjugate wholly owned by CytomX (CX-2009) and a CD71-targeting Probody drug conjugate partnered with AbbVie (CX-2029). CD166 and CD71 are among cancer targets that are considered to be inaccessible to conventional antibody drug conjugates due to their presence on many healthy tissues. The CytomX clinical stage pipeline also includes cancer immunotherapeutic candidates against validated targets such as our wholly owned anti-PD-L1 Probody therapeutic, CX-072, and the CTLA-4-targeting Probody therapeutics, BMS-986249 and BMS-986288, partnered with Bristol Myers Squibb. For additional information about CytomX Therapeutics, visit www.cytomx.com and follow us on LinkedIn and Twitter.
CytomX Therapeutics Forward-Looking Statements
This press release includes forward-looking statements. Such forward-looking statements involve known and unknown risks, uncertainties and other important factors that are difficult to predict, may be beyond our control, and may cause the actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied in such statements. Accordingly, you should not rely on any of these forward-looking statements, including those relating to the potential benefits, safety and efficacy or progress of CytomX’s or any of its collaborative partners’ product candidates, the potential benefits or applications of CytomX’s Probody platform technology, CytomX’s ability to develop and advance product candidates into and successfully complete clinical trials, including the ongoing and planned clinical trials of CX-2009 and CX-2029, and the timing of the commencement of clinical trials and other development milestones. Risks and uncertainties that contribute to the uncertain nature of the forward-looking statements include: the unproven nature of CytomX’s novel Probody Platform technology; CytomX’s clinical trial product candidates are in the initial stages of clinical development and its other product candidates are currently in preclinical development, and the process by which preclinical and clinical development could potentially lead to an approved product is long and subject to significant risks and uncertainties, including the risk that the COVID-19 worldwide pandemic may continue to negatively impact the business, research and clinical operations of CytomX or its partners, including the development of preclinical drug candidates due to delays in and disruption of research activities and the development of clinical drug candidates due to delays in or disruption of clinical trials, including impacts on the enrollment of patients in clinical trials or other clinical trial disruptions; the possibility that the results of early clinical trials may not be predictive of future results; the possibility that CytomX’s clinical trials will not be successful; the possibility that current preclinical research may not result in additional product candidates; CytomX’s dependence on the success of CX-2009, CX-2029, BMS-986249, BMS-986288, and CX-072; CytomX’s reliance on third parties for the manufacture of the company’s product candidates; and possible regulatory developments in
Probody is a
Opdivo is a registered trademark of Bristol Myers Squibb.
Investor and Media Contact:
Stern Investor Relations
stephanie.ascher@sternir.com
212-362-1200
CONDENSED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except share and per share data)
(Unaudited)
| Three Months Ended | Nine Months Ended | |||||||||||||||
| 2020 | 2019 | 2020 | 2019 | |||||||||||||
| Revenues | $ | 17,788 | $ | 10,712 | $ | 83,989 | $ | 49,210 | ||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 24,049 | 27,967 | 90,929 | 95,178 | ||||||||||||
| General and administrative | 8,634 | 8,463 | 26,886 | 27,548 | ||||||||||||
| Total operating expenses | 32,683 | 36,430 | 117,815 | 122,726 | ||||||||||||
| Loss from operations | (14,895 | ) | (25,718 | ) | (33,826 | ) | (73,516 | ) | ||||||||
| Interest income | 200 | 1,997 | 1,730 | 6,854 | ||||||||||||
| Other income (expense), net | (15 | ) | 22 | 1 | (126 | ) | ||||||||||
| Loss before income taxes | (14,710 | ) | (23,699 | ) | (32,095 | ) | (66,788 | ) | ||||||||
| Benefit from income taxes | — | — | (13,911 | ) | (6 | ) | ||||||||||
| Net loss | $ | (14,710 | ) | $ | (23,699 | ) | $ | (18,184 | ) | $ | (66,782 | ) | ||||
| Net loss per share, basic and diluted | $ | (0.32 | ) | $ | (0.52 | ) | $ | (0.40 | ) | $ | (1.47 | ) | ||||
| Shares used to compute net loss per share, basic and diluted | 46,195,121 | 45,418,053 | 45,992,786 | 45,294,593 | ||||||||||||
| Other comprehensive income (loss): | ||||||||||||||||
| Unrealized gain (loss) on short-term investments, net of tax | (63 | ) | (99 | ) | (104 | ) | 192 | |||||||||
| Impact of adoption of new accounting pronouncement | — | — | — | 11 | ||||||||||||
| Comprehensive loss | $ | (14,773 | ) | $ | (23,798 | ) | $ | (18,288 | ) | $ | (66,579 | ) | ||||
CONDENSED BALANCE SHEETS
(in thousands, except share and per share data)
| 2020 | 2019 | |||||||
| (Unaudited) | (1) | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 176,810 | $ | 188,425 | ||||
| Short-term investments | 144,266 | 107,720 | ||||||
| Accounts receivable | 543 | 13 | ||||||
| Income tax receivable | 13,061 | — | ||||||
| Prepaid expenses and other current assets | 5,625 | 7,177 | ||||||
| Total current assets | 340,305 | 303,335 | ||||||
| Property and equipment, net | 7,190 | 7,372 | ||||||
| Intangible assets, net | 1,203 | 1,312 | ||||||
| 949 | 949 | |||||||
| Restricted cash | 917 | 917 | ||||||
| Operating lease right-of-use asset | 23,239 | 25,382 | ||||||
| Other assets | 1,379 | 2,015 | ||||||
| Total assets | $ | 375,182 | $ | 341,282 | ||||
| Liabilities and Stockholders' Equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 3,643 | $ | 4,158 | ||||
| Accrued liabilities | 23,945 | 30,051 | ||||||
| Deferred revenue, current portion | 74,445 | 51,381 | ||||||
| Total current liabilities | 102,033 | 85,590 | ||||||
| Deferred revenue, net of current portion | 202,560 | 178,858 | ||||||
| Operating lease liabilities - long term | 22,525 | 24,871 | ||||||
| Other long-term liabilities | — | 850 | ||||||
| Total liabilities | 327,118 | 290,169 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders' equity: | — | — | ||||||
| Convertible preferred stock, |
— | — | ||||||
| Common stock, |
1 | 1 | ||||||
| Additional paid-in capital | 483,524 | 468,285 | ||||||
| Accumulated other comprehensive (loss) income | (47 | ) | 57 | |||||
| Accumulated deficit | (435,414 | ) | (417,230 | ) | ||||
| Total stockholders' equity | 48,064 | 51,113 | ||||||
| Total liabilities and stockholders' equity | $ | 375,182 | $ | 341,282 | ||||
__________________
(1) The condensed balance sheet as of
Source: CytomX Therapeutics Inc.
